How Do Electrons in the Same Atom Differ
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. How does molecule shape change with different numbers of bonds and electron pairs.

Online Activity Determining How Many Protons Electrons And Neutrons In An Atom Based On The Periodic Science Chemistry Teaching Science Homeschool Science
The net magnetic moment of an atom is equal to the vector sum of orbital and spin magnetic moments of all electrons and the nucleus.
. The magnetic moment of the nucleus is negligible compared with that of the electrons. In an atom the number of protons and electrons is always the same and the number of protons and neutrons is also usually the same. However when a neutron is added to the same atom it becomes an isotope or a heavier form of that atom.
When a proton is added to an atom it forms a new element. Explore molecule shapes by building molecules in 3D. Protons and neutrons have about the same mass.
Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore the number of electrons in neutral atom of Argon is 18.
Find out by adding single double or triple bonds and lone pairs to the central atom. Then compare the model to real molecules. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.
The magnetic moments of the electrons that occupy the same orbital so called paired electrons cancel each other out. Therefore the number of electrons in neutral atom of Phosphorus is 15.

What Is Electricity What Is Electricity Electronics Projects Diy Electricity

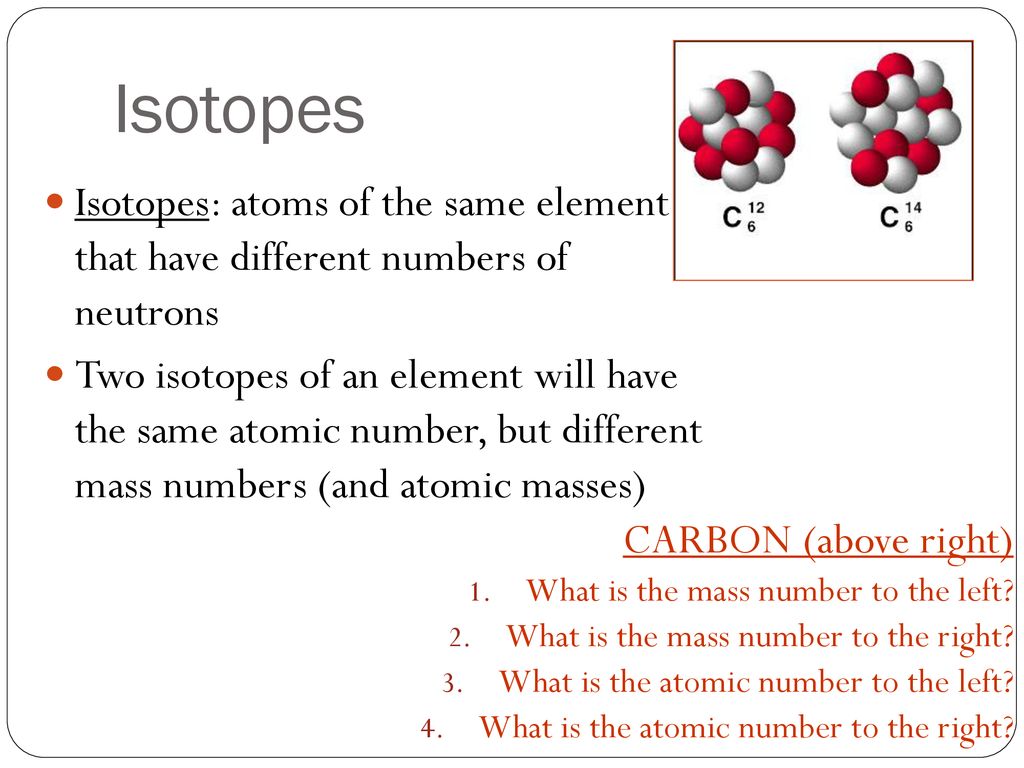
Isotopes And Ions Do Now Explain How Atoms Of Different Elements Differ From One Another Give A Specific Example Ppt Download
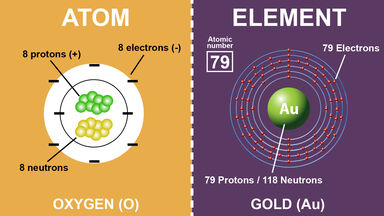
No comments for "How Do Electrons in the Same Atom Differ"
Post a Comment